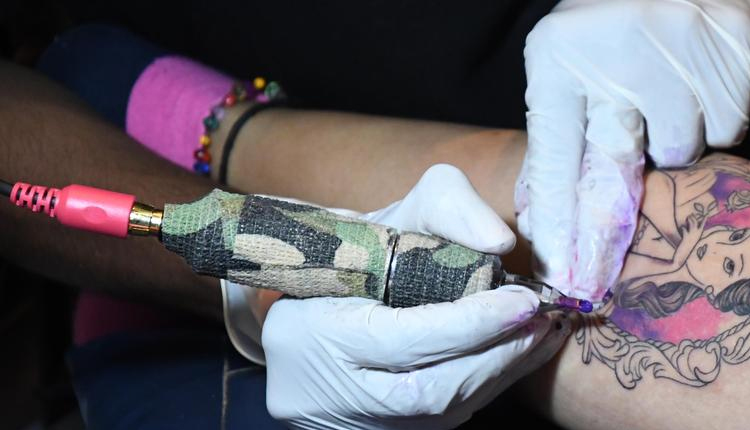
In response to complaints, officials of the Karnataka Food Safety and Drug Administration Department had collected samples for testing. Among the contents of the reports were heavy metals that were hazardous, selenium, chromium, platinum, and arsenic, which we know cause many bacterial, viral, and fungal infections, including skin diseases.
The absence of regulations around tattoo ink in India was described as “alarming” by Minister Rao. There is no standard for tattoo ink at the Bureau of Indian Standards (BIS) level; it is not a subject of any provision of the Drugs and Cosmetics Act, he alleged. This implies the absence of laws dictating the permissible chemical composition and the guidelines for its application.
Rao said that there needs to be a proper protocol regarding tattoo inks, which is currently not the case in India. Taking the matter seriously, he stated that the state government will appeal to the central authorities. An official letter from the Food Safety and Drug Commissioner will soon be sent to DCGI, inviting it to set regulatory standards based on the BIS guidelines. Doing so would distinguish safe tattoo inks from unsafe ones, and it would also allow public health officials to regulate inks through law.
Speaking about the risks of tattoo ink, Rao elaborated: The chemicals are known to penetrate the skin and therefore could be harmful. While some of the chemicals remain on the surface, others are absorbed into the skin, potentially leading to health-related issues over time. And for that reason, we need to make sure we monitor this one very closely to prevent long-term health risks to people.
The minister disclosed that the Karnataka Food Safety and Drug Administration Department has been involved in several recent enforcement actions. In January, 106 out of 1,133 drug samples failed quality tests, while the remaining samples met the required standards. Some 58 of more than 1,840 drug samples did not meet quality standards in February. Hence, 75 cases of FOR violations involving substandard and poor-quality drugs have been registered under the Drugs and Cosmetics Act, 1940.
The state initiated a special campaign in January to remove these substandard drugs from the market, having identified them earlier. Drugs worth around ₹17 lakh were, however, pulled out in the process. Rao said, “In December 2024, of the total number of 262 cosmetic samples analysed, 120 had quality standards, while a total of 142 are still in the process of analysis.”
Importantly, a separate anti-drug operation was started in January of this year to control misuse of the NDPS (Narcotic Drugs and Psychotropic Substances) Act. This drive involved the inspection of 488 medical stores, revealing 400 violations. As a result, we issued show-cause notices to the companies at fault, cancelled 231 licenses, and revoked 3 licenses.
In a campaign conducted across the state from February 17 to 19, called“ Was there a prescription before use?” where the officials kept a strict check on the misuse and sale of antibiotics, 52 medical stores were found to be dispensing antibiotics without a prescription, said the officials. We are currently investigating these cases and will take necessary action.
As far as the Ringer Lactate Solution matter is concerned, Rao said 113 samples were found unfit. Consequently, various courts have filed nine lawsuits and approved legal action against 36 firms that provided faulty solutions. The health department plans to implement a computerised system to improve supervision and tackle substandard drugs more effectively.


Comments are closed.